What is Levera RTU?
Levera RTU is ready to use injection of LEVETIRACETAM available as infusion bottle.
In which strength/ strengths Levera RTU available?
Levera RTU available in three strengths:
- Levetiracetam in 0.54% Sodium Chloride Injection, 1500 mg/100 mL Infusion Bottle
- Each 100 mL contains:
- Levetiracetam IP 1500 mg
- Water for Injections IP Q.S.
- Levetiracetam in 0.75% Sodium Chloride Injection, 1000 mg/100 mL Infusion Bottle
- Each 100 mL contains:
- Levetiracetam IP 1000 mg
- Water for Injections IP Q.S.
- Levetiracetam in 0.82% Sodium Chloride Injection, 500 mg/100 mL Infusion Bottle
- Each 100 mL contains:
- Levetiracetam IP 500 mg
- Water for Injections IP Q.S.

What are the indications for Levera RTU?
Levera RTU is an antiepileptic drug indicated as adjunctive therapy with the following types of seizures when oral administration is temporarily not feasible:
- Partial onset seizures in patients 1 month of age and older with epilepsy
- Myoclonic seizures in patients 12 years of age and older with juvenile myoclonic epilepsy
- Primary generalized tonic-clonic seizures in patients 6 years of age and older with idiopathic generalized epilepsy
What is the dosage of Levera RTU??
Partial Onset Seizures: Initial dose is 1000 mg/day, divided as 500 mg twice daily. Increase dose as needed and tolerated in increments of 1000 mg/day, every 2 weeks to a maximum recommended daily dose of 3000 mg/Day.
Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy: Initial dose is 1000 mg/day, divided as 500 mg twice daily. Increase dose by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg/Day. The effectiveness of doses lower than 3000 mg/day has not been studied.
Primary Generalized Tonic-Clonic Seizures: Initial dose is 1000 mg/day, divided as 500 mg twice daily. Increase dose by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg/Day . The effectiveness of doses lower than 3000 mg/day has not been adequately studied.
Switching to Intravenous Dosing: Initial total daily intravenous levetiracetam dose regimen should be equivalent to total daily dose and frequency of oral levetiracetam.
Switching to Oral Dosing: Give equivalent daily dose and frequency of oral as intravenous levetiracetam.
Which important measures should be considered while using Levera RTU?
General Information for Levera RTU:
- For intravenous infusion only.
- Do not dilute prior to its use.
- Any unused portion of the Levetiracetam in Sodium Chloride Injection contents should be discarded.
- Administer dose-specific 100 mL infusion bottle intravenously over 15-minutes
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
In how much duration Levera RTU should be administered?
Dose-specific 100 mL infusion bottle of Levera RTU should be administered intravenously over 15-minutes
| LEVETIRACETAM Dose | Equivalent Levera RTU | Concentration | Volume | Infusion Time |
| 500 mg | LEVERA RTU 500 | Levetiracetam in 0.82% Sodium Chloride | 100 ml | 15 minutes |
| 1000 mg | LEVERA RTU 1000 | Levetiracetam in 0.75% Sodium Chloride | 100 ml | 15 minutes |
| 1500 mg | LEVERA RTU 1500 | Levetiracetam in 0.54% Sodium Chloride | 100 ml | 15 minutes |
Why it should be administered over 15 minutes?
In a bioequivalence study, levetiracetam 1500 mg was infused over 15 minutes. The selected infusion rate provided plasma concentrations of levetiracetam at the end of the infusion period similar to those achieved at Tmax after an equivalent oral dose.
It is demonstrated that levetiracetam 1500 mg intravenous infusion is equivalent to levetiracetam 3 x 500 mg oral tablets.
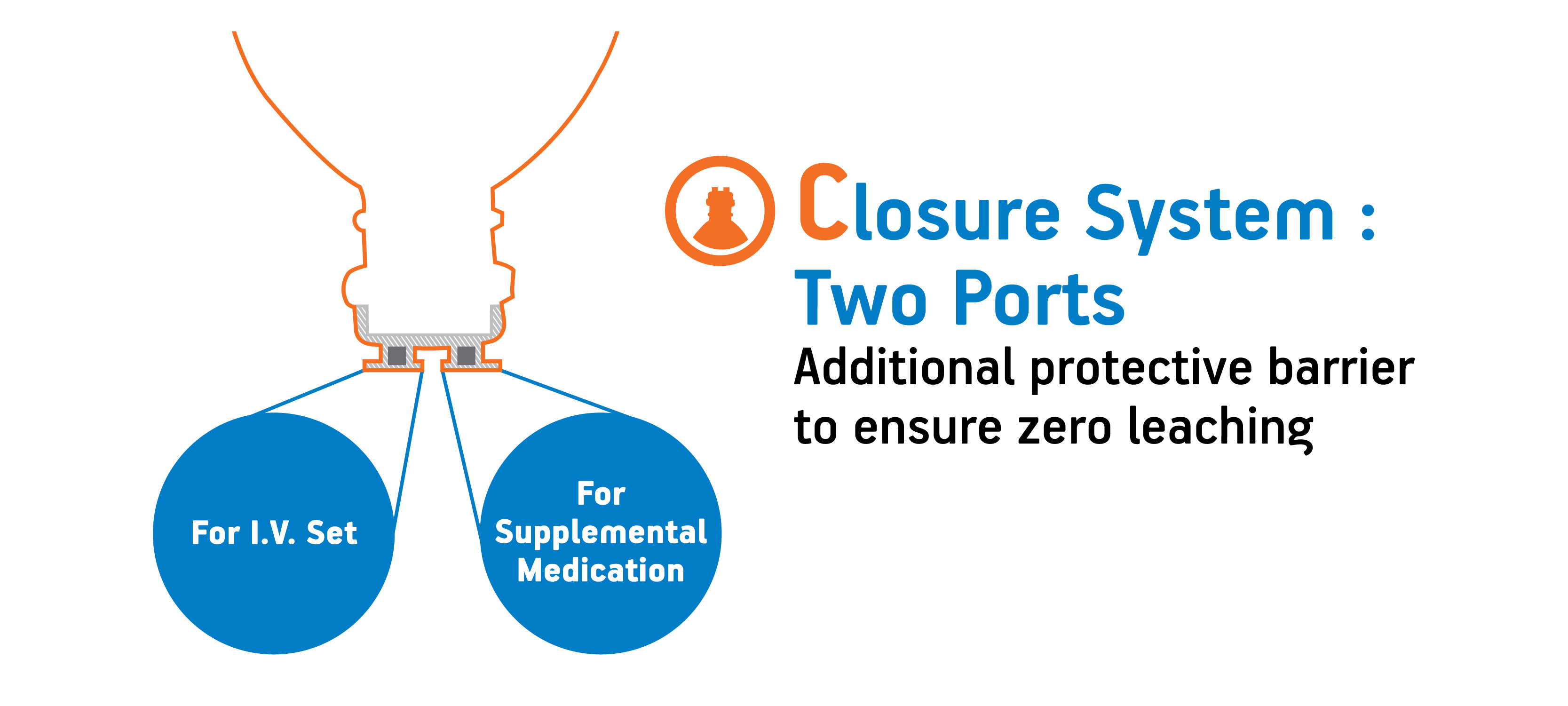
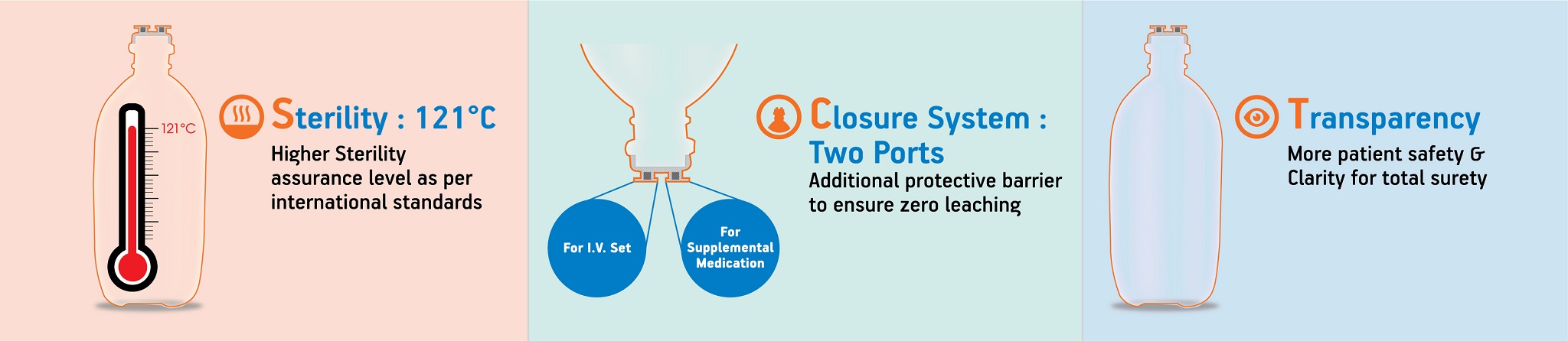
What are the advantages of two port closure system?
Advantages of two port closure system:
- One for IV Set.
- The second for supplemental medication.
- There is an additional protective barrier between the solution and the head.
- Latex-free elastomer is used for the cap.
- The closure system is 100% leak-proof.
- Multiple spiking of the head is possible.
What is the special feature of port?
It has been frequently observed by the paramedics that in preparing intravenous infusion leakage from nipple-head BFS (FFS) bottle of I.V. fluids, after spike of I.V. set is inserted into the container, leakage is a common occurrence. This results in the wastage of I.V. fluids. This causes a host of problems like financial loss, I.V. fluid spillage on operation theatre floor, and importantly, error in calculating administered I.V. fluid (very critical in pediatric patients). The gravity of this problem in India where often patient workload outnumbers the available resources and quality controls are not so stringent. This problem has been completely eliminated in Levera RTU infusion bottle.
Which other antiepileptic drugs can be combined with Levera RTU 2nd Port?
Levera RTU can be mixed with following other Antiepileptic Drugs
- Lorazepam
- Diazepam
- Valproate sodium
There are no data to support the physical compatibility of levetiracetam injection with antiepileptic drugs that are not listed above.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Product with particulate matter or discoloration should not be used.
What are the benefits of Levera RTU infusion bottle?
Levera RTU infusion bottle fulfils SCT Pharma standards recommended around the world.
How to store Levera RTU?
Levera RTU is a clear, colourless, sterile solution infusion bottle should be stored at 20° to 25°C (68° to 77°F).
What is self-life of Levera RTU?
2 Years
